(Department of Chemistry, Institute of Pure and Applied Sciences, University of Tsukuba)
要旨
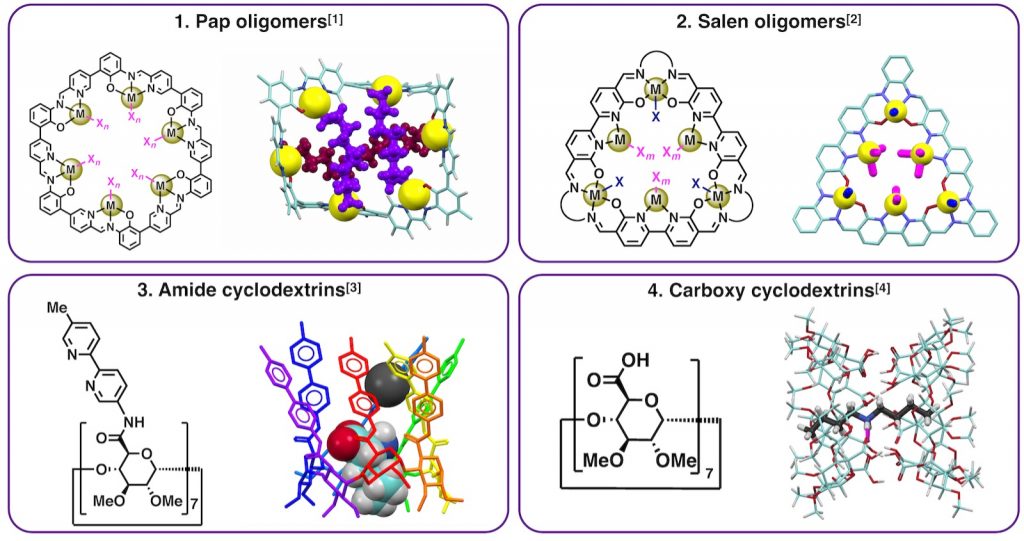
Natural receptor proteins can selectively bind substrates within a pocket surrounded by multiple amino acid residues. During the recognition process, relatively weak intermolecular interactions, such as hydrogen bonds, act synergistically to achieve high selectivity. In contrast, it has been challenging for synthetic receptors to achieve precise molecular binding by arranging multiple interaction sites in an unsymmetrical manner.
Our group has developed novel macrocyclic receptors with precise recognition capabilities based on two key strategies: (A) the assembly of metal coordination sites and (B) the desymmetrization of molecular components. Specifically, I will present our recent progress on cyclic oligomers of chelate complexes capable of multipoint coordination, and cyclodextrin derivatives equipped with hydrogen bonding sites such as amide and carboxyl groups.
References
- (a) Commun. 2017, 8, 129. (b) Chem. Commun. 2019, 55, 2421–2424. (c) Eur. J. Inorg. Chem. 2021, 308–313. (d) Inorg. Chem. 2023, 62, 12886–12894. (e) Chem. Commun. 2024, 60, 1281–1284.
- (a) Am. Chem. Soc. 2019, 141, 6462–6467. (b) Inorg. Chem. 2019, 58, 7863–7872. (c) Inorg. Chem. 2024, 63, 12697–12702. (d) Chem. Commun. 2025, 61, 921–924.
- (a) Commun. 2019, 55, 3872–3875. (b) Chem. Lett. 2020, 49, 493–496. (c) Angew. Chem. Int. Ed. 2021, 60, 3080–3086. (d) Chem. Sci. 2025, 16, 171–181.
- (a) In revision.
世話人: 重田育照、Kowit Hengphasatporn